Good documentation drives good clinical research. As the industry saying goes, “If it isn’t documented, it doesn’t exist.” But monitors often discover a lack of adequate source documentation during inspections. 6 out of 10 warning letters issued to clinical investigators by the FDA cited:
Failure to maintain adequate and accurate case histories that record all observations and other data pertinent to the investigation on each individual administered the investigational drug or employed as a control in the investigation
Because Florence helps over 10,000 research sites manage eRegulatory files, we prioritize ALCOA-C standards and additional European Medicines Agency (EMA) standards. We use these guidelines to design our platform, workflows, and SOPs and to train research sites.
Let’s Talk Source
When you think about ALCOA-C, you first need a clear definition of source data. According to the FDA, source documentation is any medical record or form kept for a participant before, during, or after the clinical trial procedure. The FDA and other regulatory bodies prioritize the quality of source documents because these documents let monitors investigate and validate trial data.
Good documentation also proves that the trial respected ethics and participants’ well-being.
The FDA became the first body to introduce standards for source documents with ALCOA-C. The EMA followed suit and added a few additional letters to the acronym. (Hey, regulatory bodies love standards!)
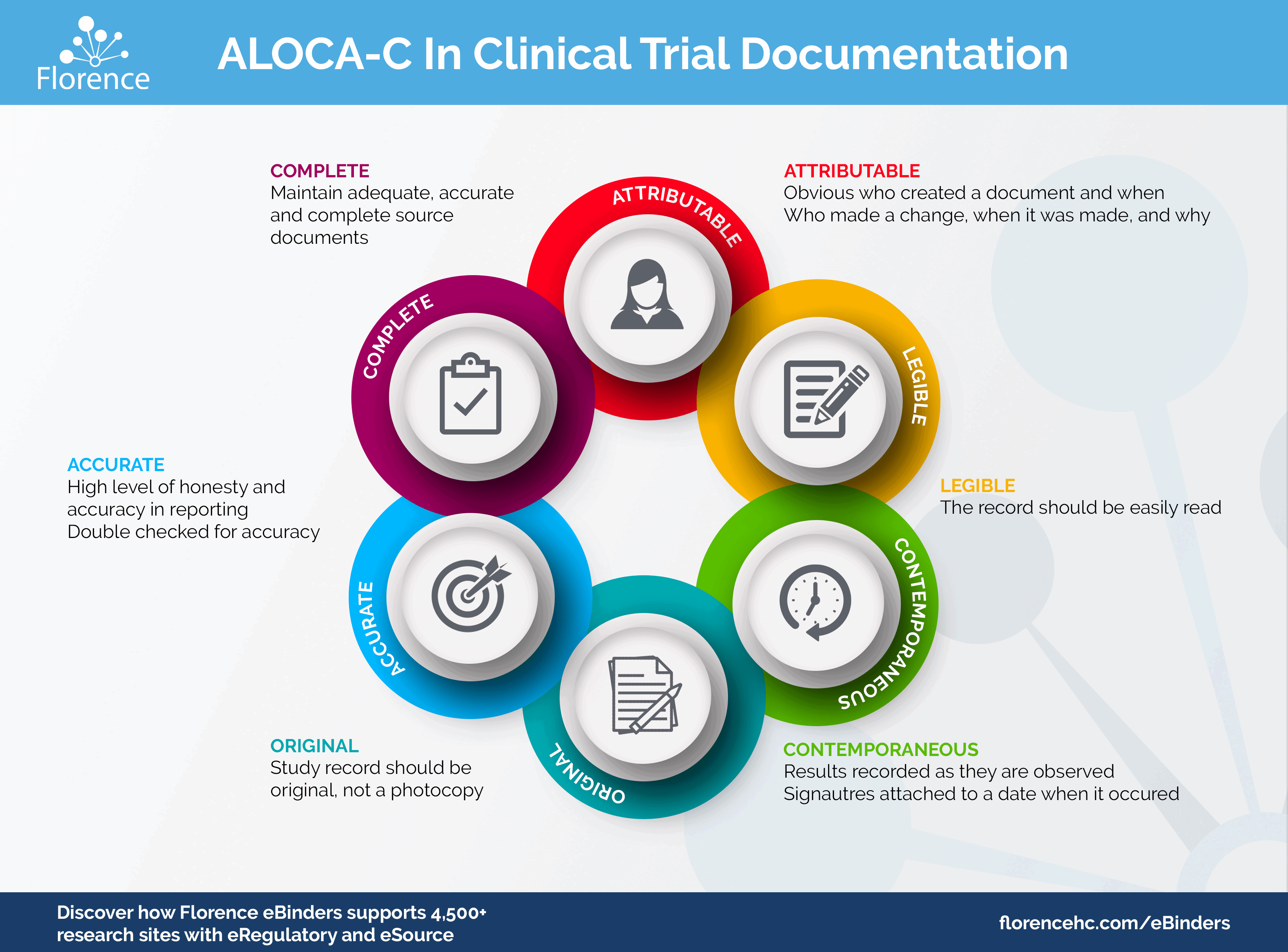
To help you understand the basics of ALCOA-C, we’ve put together this infographic highlighting what you need to know about each letter:
ALCOA-C
Let’s go deeper into ALCOA-C and the other standards introduced by the EMA:
Attributable
Monitors should be able to tell who recorded data or created a document. The document also must have an audit trail showing who made a change, why, and when.
To make sure you’re following these standards, your research team must have a working Delegation of Authority (DOA) Log. Florence eBinders simplifies DOA Logs by making them fully electronic and by including Part-11 compliant eSignatures.
When your research team moves to eRegulatory/eSource, you’ll also benefit from automated and always-on audit trails. Site staff, monitors, and inspectors can easily see when a document was viewed, created, edited, or signed. They can also see who performed each action and why they performed the action.
Legible
We’ve all encountered documents with text that’s impossible to make out. Is that a 6 or an 8? Many research sites run into these compliance risks when managing handwritten regulatory and source documents. With eRegulatory and eSource platforms, you can lower your site’s risk of illegible documents. Electronic forms and direct integrations with the EMR/EHR keep data legible and consistent.
Contemporaneous
Clinical research professionals should record results when they observe them, and signatures must come with a date. If you can’t enter a clinical outcome as soon as it happens, you need to explain why it wasn’t recorded right away in your documentation. An electronic system with audit trails makes it easy to enter data quickly and track when data was entered.
Original
You must maintain original study documents or certified copies, not informal photocopies. Fortunately, online systems allow you to create or upload original documents and create certified copies.
Accurate
The FDA and other regulatory bodies require completely accurate documents and data. Clinical research professionals must check the data they’ve entered on paper or in an electronic system to make sure they’ve recorded every data point correctly. Integrating your clinical research software with your EHR or EMR can improve your accuracy, since you won’t have to re-enter data.
Complete
Keep your documents complete. Don’t wait a few days or weeks to record data if you want to avoid audit findings.
Additional governing body standards not covered in ALCOA-C:
Enduring
Your documents should be durable and easy to access and read long after they’re created. Electronic documents work much better in this regard than paper ones. Florence eBinders provides easy archiving for completed studies and the ability to download complete binders if needed.
Available and accessible
We’ve all been through monitoring visits where locating a file feels like a scavenger hunt. But the EMA specifies that physicians providing treatment and monitors performing audits or inspections must have easy access to documents. Florence eBinders enables this with simple and fully compliant monitor access.
Consistent
Your documents must demonstrate all required attributes consistently. eBinders offers form templates so you don’t have to worry about incomplete or inconsistent documents.
Credible
The data in your documents must come from reliable scientific observation. Every clinical research professional knows this, but it’s important to make sure you can store your source data as observations happen, without needing to re-enter it in multiple systems.
Corroborated
Your trial data should be backed up by additional evidence. When monitors review source documents and data, the documents gathered must support one another.
Want to learn more about the regulatory standards around digital research? Check out our Complete Guide to eRegulatory and eSource!