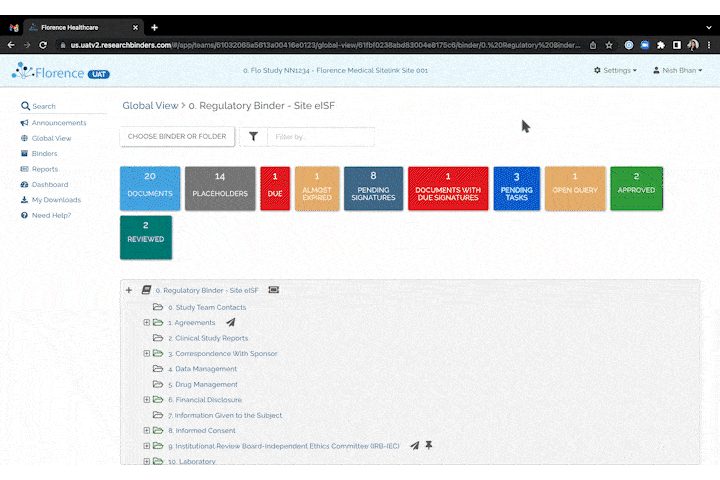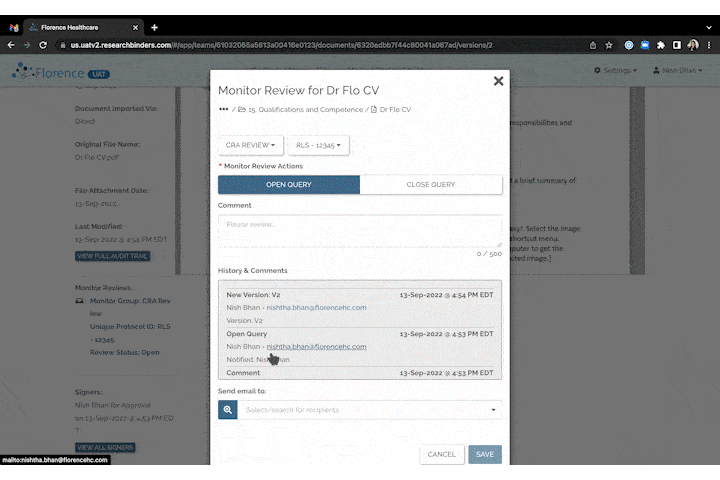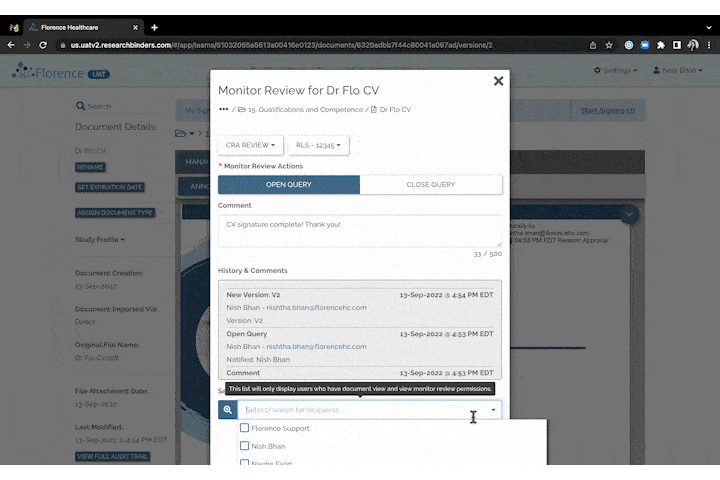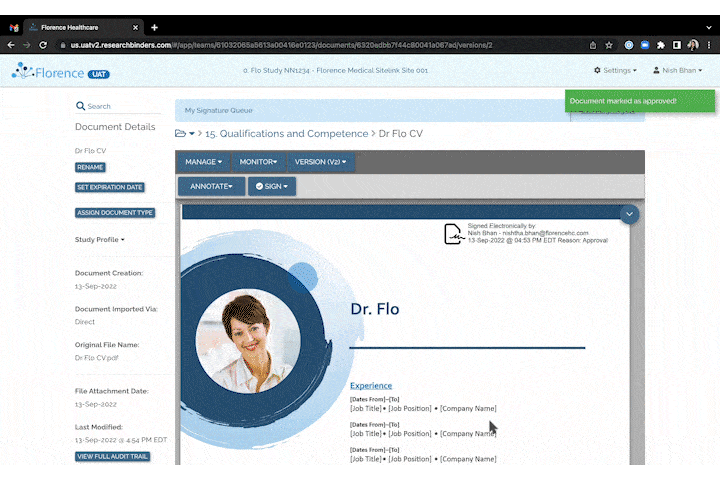
Invite to SiteLink Accelerator Program
You’re invited to test drive the industry’s largest Site Enablement Platform.
Learn first-hand why sponsors and CROs are rapidly deploying SiteLink™ to create a direct link with every site in their study using this exclusive pilot program designed for the most innovative research organizations to test the benefits with minimal risk.
Link to any Study Site for Remote Start-up, Monitoring, SDR/V, and Close-out
Sites on Florence eBinders™
A direct link with more than 18,000+ sites in 55+ countries active on Florence’s network.
Sites on other eISFs
An open API to connect with any sites eISF so you don’t disrupt their workflows.
Frontier Sites
Deploy the industry-standard eISF to “new to research” sites.
2022 SiteLink Trial Accelerator Frequently Asked Questions

SiteLink is helping Pfizer to respond to the changing environment due to COVID-19 and further progress COVID-19 research with the capability to perform remote monitoring where approved by regulatory authorities and ethics committees.

Rob Goodwin
VP and Head of Operations in Global Product Development
Pfizer
Case Study: Study Start-up
Reduce the time you spend collaborating on regulatory documents by 41%.
Case Study: Study Conduct
Overcome staffing shortages and help your CRAs monitor 2-5x more sites per week.
Why Sponsors and CROs Activate Always-on Remote Workflows with Sites on SiteLink
Make Sites Happier
When you give your sites Florence eBinders through SiteLink, you give them a platform they love. Florence eBinders is ranked #1 for clinical trial workflows by sites for ease-of-use, ease-of-setup and support.
Maximize CRA Efficiency
CRAs with remote access capabilities can monitor over 60 sites per week with SiteLink. CRA staffing bottlenecks and travel costs are reduced, and CRAs can make a greater impact on study sites.
Expand Patient Access
Deploying remote digital workflows to sites means you can expand access to a geographically dispersed site network. Active sites on Florence already are within 25 miles of 90% of the US population.
Activate Sites Fast
Coupling our existing global network of connected sites with our #1-ranked site activation team, we set up your sites in days with a 92%+ site acceptance rate on Florence eBinders™, the industry-standard eISF and Electronic Participant Binder for sites.
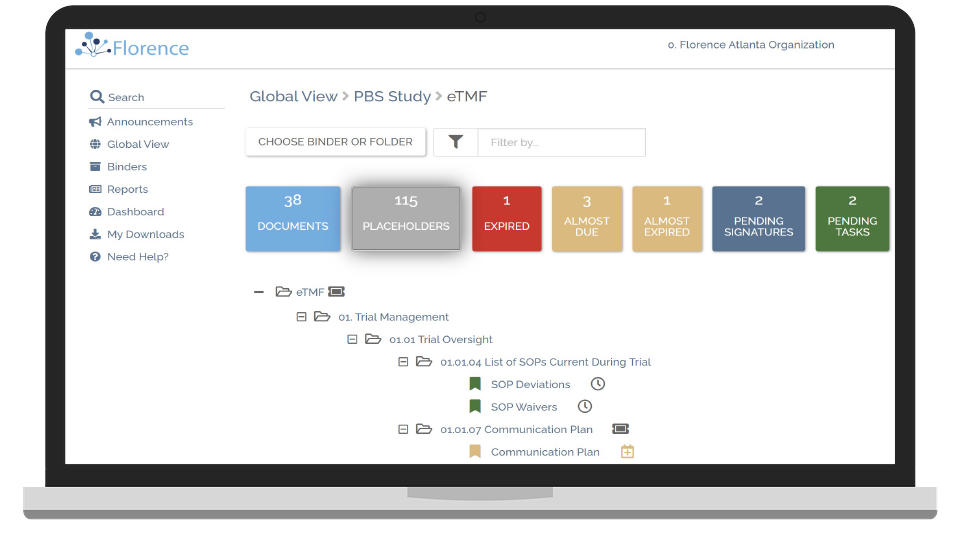
Improve eTMF Quality
Over 80% of your eTMF is generated at the trial site. By integrating SiteLink with your existing eTMF or with the Florence eTMF, you can exchange documents seamlessly. The eTMF pass rate of one customer increased from 65% to 98.7%.
Accelerate Study Timelines
Automated workflows like electronic logs, placeholders, eSignatures and quality assurance workflows reduce start-up times by 40% for most of our customers and accelerate overall study timelines to reduce time to submission.
In clinical research, compliance is crucial. We’ve got you covered globally.






We are committed to making you and your sites successful.
We are committed to making you and your sites successful.
SiteLink Workflows for Study Sponsors + CROs
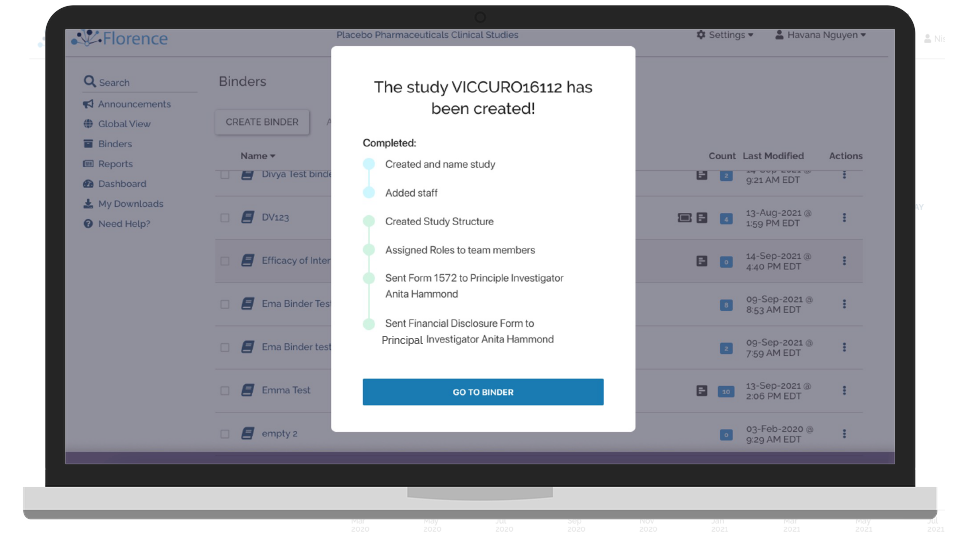
Start Up Sites Remotely
Remotely activate and start up your study sites with a complete suite of electronic binder solutions.
“92% of 140 study sites in 8 countries were activated on Florence for remote source access and monitoring in four weeks.”
VP Clin Ops, Top 3 Pharma
“Our highest performing CRAs are now ‘visiting’ 64 sites per week with remote monitoring on SiteLink, up from 2 per week.”
VP Clin Ops, Top 5 CRO
Experience 24/7 Continuous Monitoring of Sites
Access your study sites’ Electronic Investigator Site Files anytime for continuous monitoring.
Remote SDV and Remote SDR
Conduct remote source data review and remote source data verification anytime.
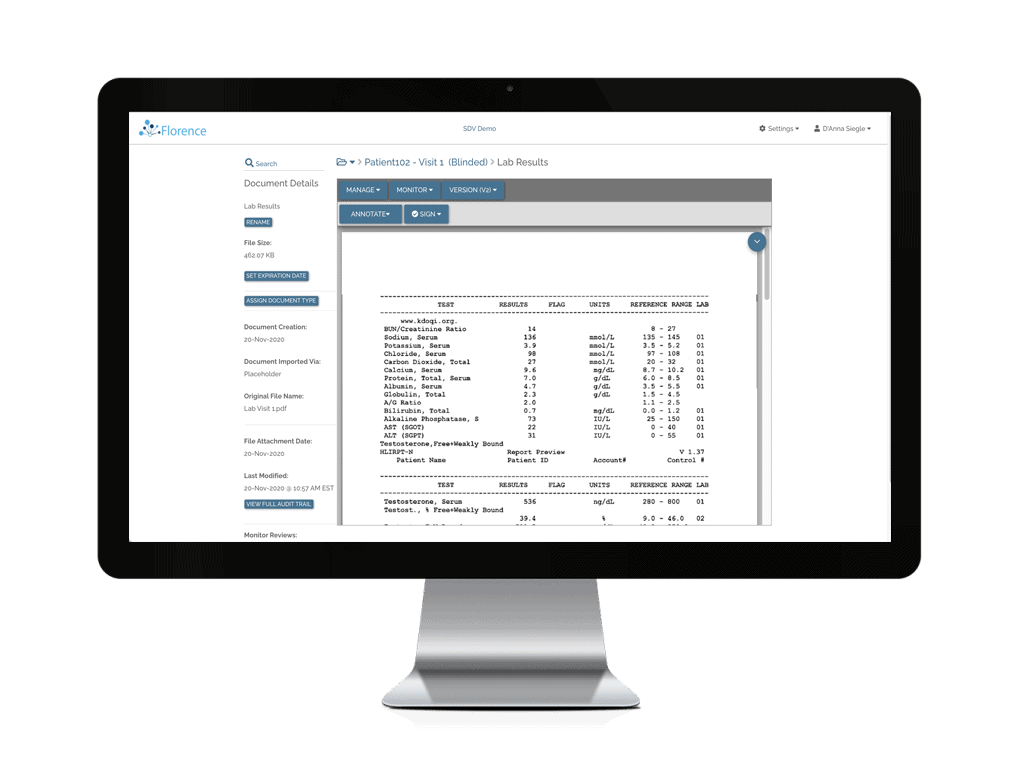
“I worked with a site that was supposed to get something done for me in 6 months, a tight timeline. They got it done in 18 days.”
Medical Device Industry Sponsor
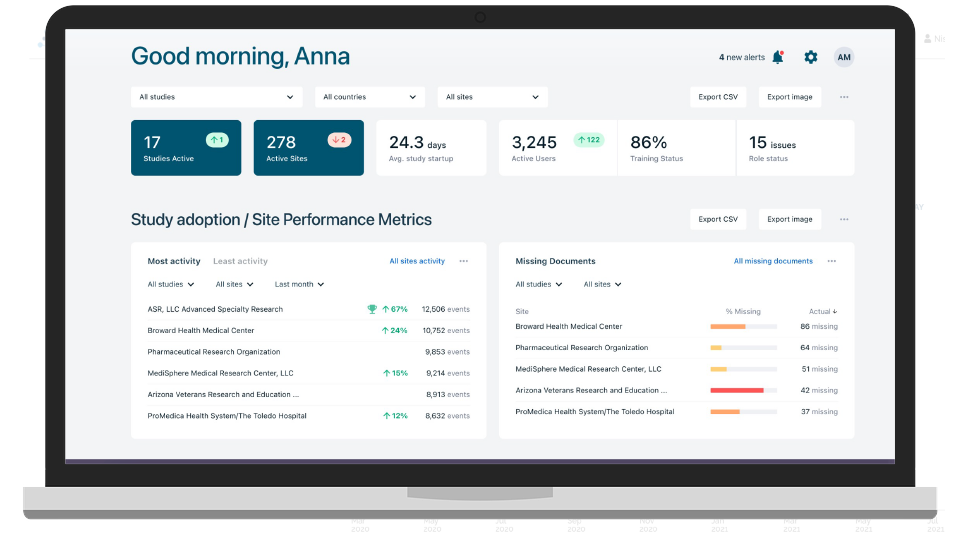
“Every day you delay a clinical trial you lose $600K. For a blockbuster drug, that’s 8M per day – that’s just a fact. Florence’s SiteLink saves us 90-100 days on a trial. You do the math.”
VP Site Management, BioPharma
Communicate and Collaborate with Sites Remotely
Enable global dashboards, compliant workflows, communications and electronic logs to remotely manage study site conduct.
Quality Control Documents and Sync Them to Your eTMF
Eliminate the need for mailing, emailing, and faxing study documents or using legacy site upload portals by directly integrating your eTMF with your sites’ eISFs.
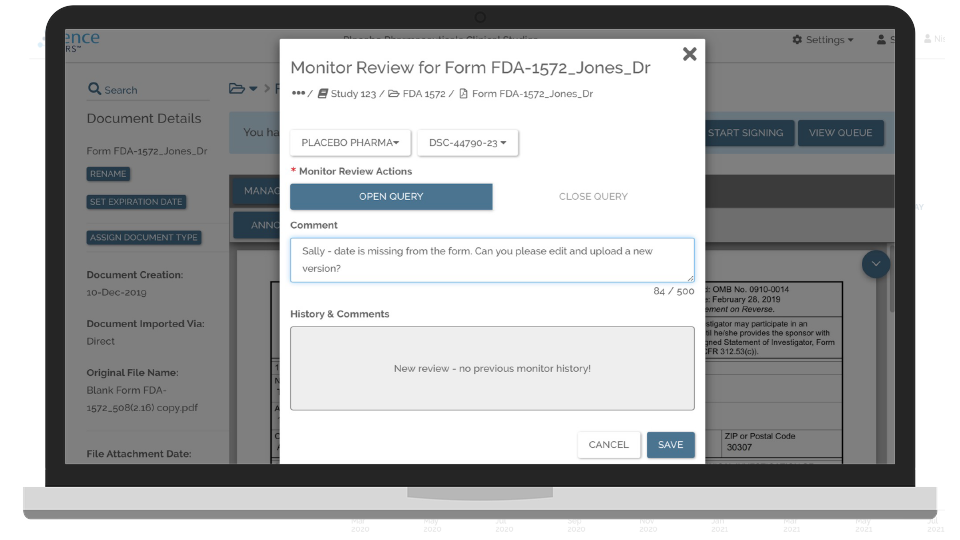
“When we start a study there is a regulatory submission … we used to be at a 65% pass rate, now with Florence we are at 99%.”
Director Regulatory, Pharma