Solutions > eBinders™
Florence eBinders™
Simplify operations.
Streamline workflows.
Integrate processes.
Digitize all of your study binder workflows with Florence eBinders™ and provide remote access for start-up, monitoring and source data review for your sponsors. eBinders is trusted by 18,000+ research sites around the globe.
How eBinders Transforms Your Operations
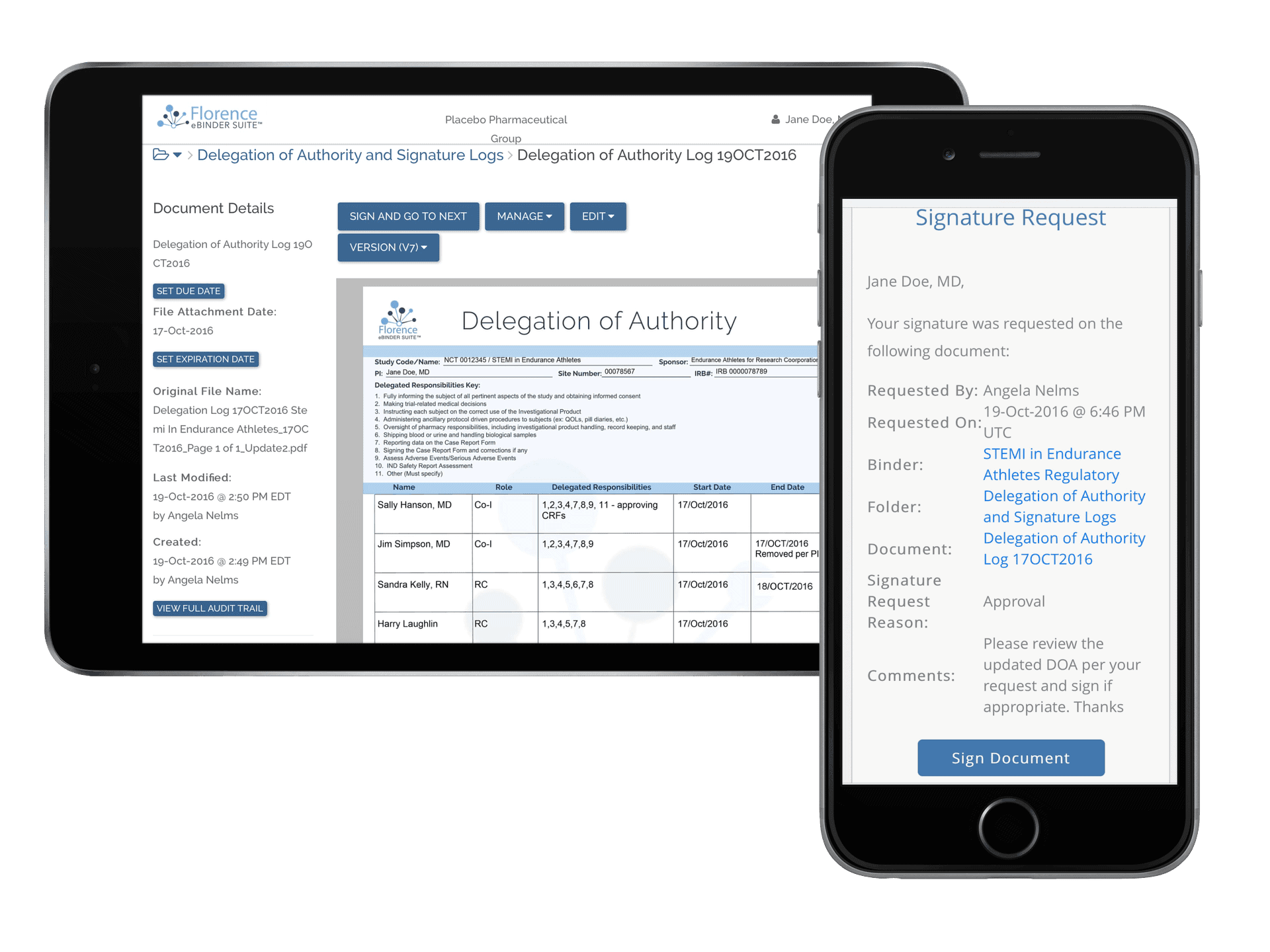
Digitize, automate, and integrate your electronic investigator site files, participant binders, and logs.
Go Digital from Start-up to Close-out
Transform your study start-up process with our all-in-one platform. Create, edit, distribute, collect, sign, and review all necessary files electronically, streamlining the process and reducing study start-up times by up to 40%. One NCI Cancer Center reduced their average time to sign from 2 weeks to just 4 hours.
Experience Flexible Workflows
eBinders lets you set up your structures and workflows the way you need while maintaining regulatory compliance. Empower remote workforces to help your team work from anywhere.
Enjoy an Easy-to-use Interface
Florence is rated the #1 clinical trial workflow platform on G2 for ease of use, ease of setup, and customer support. Even the most paper-loving PI will enjoy moving to Florence eBinders – and our industry-leading implementation and customer operations team will help you do it.
Automate Compliance
Built-in compliance features include automated audit trails, version control, extensive user permission options and in-app redaction. Plus, your team is supported by our extensive compliance program to help develop SOPs for going digital.
Enable Remote Monitoring
Give sponsor and CRO monitors secure and compliant access to your electronic investigator site file and redacted participant binder. Automate scheduling of remote visits, track monitor activity, and communicate with your monitor on follow-up items.
Customizable Electronic Logs
Improve patient safety, increase study speed, and ensure data quality with electronic clinical trial logs on Florence. Customize workflows for any log you need – Delegation of Authority Logs, Adverse Event Logs, Training Attestation Logs, Informed Consent Version Logs, Screening/Enrollment Logs, and more.
In clinical research, compliance is crucial. We’ve got you covered.






Why being rated #1 out of 190 clinical trial platforms on G2 by research sites matters for you.
Collaborating in real-time on a single document management platform helps us tackle study tasks faster and keep research on track.
Dr. Christina Brennan
VP of Clinical Research

Accelerate Study Start-up
Use intuitive workflows to get your study set up and activated fast.
Sync Source with Participant Binders
Create electronic participant binders to collect all participant source data in a single location.
Track Study Performance in Real-Time
Keep track of your entire study and identify risk areas with advanced reports and dashboards.

Enable Remote Monitoring
Equip your site to work with sponsors remotely on start-up, monitoring and source data review.
Digitize Study Logs
Migrate all of your study logs into a digital format for creation, completion and review.